The estimation of calcium in blood-serum / by John William Trevan and Henrietta Winifred Bainbridge.
- Trevan, J. W. (John William)
- Date:
- [1926?]
Licence: In copyright
Credit: The estimation of calcium in blood-serum / by John William Trevan and Henrietta Winifred Bainbridge. Source: Wellcome Collection.
1/4
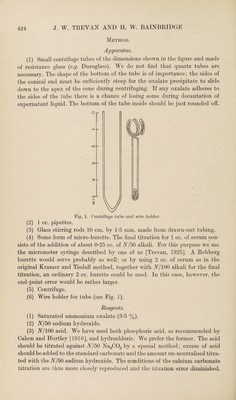
![[From THE BIOCHEMICAL JOURNAL, Vol. XX, No. 2, 1926] {All Rights reserved] \ PRINTED IN GREAT BRITAIN I...A) LVII. THE ESTIMATION OF CALCIUM IN BLOOD-SERUM. By JOHN WILLIAM TREVAN AND HENRIETTA WINIFRED BAINBRIDOE. From the Wellcome Physiological Research Laboratories, Langley Court, Beckenham, Kent. {Received March 11th, 1926.) The method for the estimation of calcium in blood-serum devised by Kramer and Tisdall [1921] has been extensively used and, it is generally agreed, gives reproducible results in expert hands. Clark and Collip [1925], however, em¬ phasise that the details of the method must be followed with meticulous accuracy or discrepant results will be obtained. They attribute the main source of error to the slight solubility of calcium oxalate and are of opinion that traces of ammonium oxalate, used for washing the precipitate, remain, and, if close adherence to the original method is maintained, tend to com¬ pensate for the error due to solution of the calcium oxalate. In consequence too much washing, or too little, will give inaccurate results. The original method involves titration of the precipitated oxalate with A/100 potassium permanganate. One of us, apart from an instinctive aversion from perman¬ ganate titrations, finds it extremely difficult to decide when the end point is reached although the other author can, by the exercise of some patience, obtain reproducible titration figures. It occurred to us that by the conversion of the oxalate into carbonate the use of permanganate could be avoided, whilst the amount of carbonate could be estimated by acidimetric methods. Hamilton [1925] has used a similar modification of the Kramer and Tisdall method which was published before the working out of our method was com¬ pleted, but the details of the method we use make a considerable difference in the time necessary to complete an estimation, as compared with that required by the method used by Hamilton. The method also has the advantage that the calcium oxalate need not be washed with water at any stage, saturated ammonium oxalate in which calcium oxalate is absolutely insoluble, being used instead. Any excess of ammonium oxalate is decomposed and driven off as carbonate when the final heating stage is reached.](https://iiif.wellcomecollection.org/image/b30625993_0001.jp2/full/800%2C/0/default.jpg)